Specifications
Before using the LabPad® INR PT/INR monitoring system without outside aid, you must consult your physician in order to determine a target value as well as a therapeutic range with a low and high value. Your physician will also provide your INR test frequency.
LabPad® INR
Operating conditions
Location with sufficient lighting
Ambient temperature 15-32°C
Relative humidity level < 85%
Storage conditions
Transport conditions
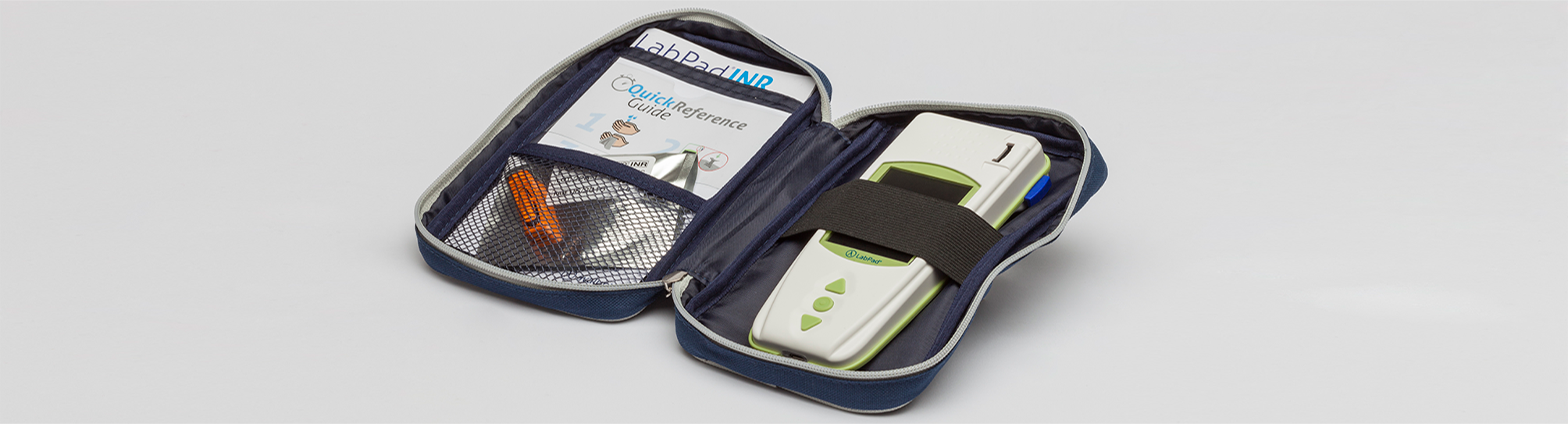
In the carrying bag supplied with the device
Analysis time
User interface
Color display
Measuring range
PT 7.2 -72 seconds
QT 10-110%
Quality controls
USB interface
Battery recharge
Battery charger
Power pack 100-240v, 50-60Hz, input 0.2A, output 1.0 A
Battery autonomy
3 months without use
Size
W 7.21 cm
H 3.18 to 2.26 cm
Weight
Memory
Power off
Can be set from the “Settings” menu
Bluetooth
Can be set from the “Settings” menu
Beep
Microcuvette Tsmart® INR
Operating conditions
At ambient temperature before use
Within 10 min after opening the pouch
Storage conditions
Between 15 and 25°C until the expiry date indicated on the pouch
Possible refrigeration